Insana Lab: Ultrasonic Imaging - The University of Illinois at Urbana-Champaign
Project 4: Atherosclerosis and the role of cell adhesion and blood flow dynamics
Jean K. Tsou [jktsou.ucdavis.edu]
 Fig 1.
Cardiovascular disease from atherosclerosis is a lead cause of mortality
in the United States. Nascent atheromas commonly form in regions of arterial branching and curvature,
such as the infra-renal abdominal aorta, carotid sinus, coronary, iliac, and superficial femoral
arteries (Fig. 1a)(G Helmlinger 1995). Atherogenesis preferentially occurs in vessel walls near
bifurcations and points of blood flow recirculation and stasis, where the magnitude of wall shear
stress is significantly lower and exhibits directional changes (Fig. 1b)(AM Malek 1999). Atherogenesis
involves dysfunction of the vascular endothelium, the monolayer of cells lining the inner surfaces
of all blood vessels. It is initiated when white blood cells pass through the endothelial cell (EC)
lining to enter the intimal space and transform into macrophages. With the accumulation of macrophages
and lipid in the intima, the plaque grows into lumen perturbing and eventually reducing blood flow.
Also small blood clots forming near the flow disturbance may be shed to cause a stroke or pulmonary
embolism. (Fig. 1c)
Fig 1.
Cardiovascular disease from atherosclerosis is a lead cause of mortality
in the United States. Nascent atheromas commonly form in regions of arterial branching and curvature,
such as the infra-renal abdominal aorta, carotid sinus, coronary, iliac, and superficial femoral
arteries (Fig. 1a)(G Helmlinger 1995). Atherogenesis preferentially occurs in vessel walls near
bifurcations and points of blood flow recirculation and stasis, where the magnitude of wall shear
stress is significantly lower and exhibits directional changes (Fig. 1b)(AM Malek 1999). Atherogenesis
involves dysfunction of the vascular endothelium, the monolayer of cells lining the inner surfaces
of all blood vessels. It is initiated when white blood cells pass through the endothelial cell (EC)
lining to enter the intimal space and transform into macrophages. With the accumulation of macrophages
and lipid in the intima, the plaque grows into lumen perturbing and eventually reducing blood flow.
Also small blood clots forming near the flow disturbance may be shed to cause a stroke or pulmonary
embolism. (Fig. 1c)
|
|
 Fig 2a illustrates the process of atherogenesis in the cellular level. Abnormal
wall shear stress patterns and the presence of cytokines and other factors act to stimulate the
ECs to produce an inflammatory response that includes adhesion molecules (E-selectin, intravascular
adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1)). The ligands and integrins
on the monocytes bind to the adhesion molecules expressed on the endothelial cell surface and
transmigrate into the subendothelial space. The monocytes then transform into macrophages and bind
to highly-oxidized low-density lipoprotein (LDL) to form foam cells. The death of foam cells leaves
behind a growing mass of extracellular lipids and other cell debris along with the accumulation of
smooth muscle cells, which later form fibrous plaques. Vulnerable plaques with thin fibrous caps
have high risk to be ruptured and form thrombus inside the lumen.(Lusis 2000)
Fig 2a illustrates the process of atherogenesis in the cellular level. Abnormal
wall shear stress patterns and the presence of cytokines and other factors act to stimulate the
ECs to produce an inflammatory response that includes adhesion molecules (E-selectin, intravascular
adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1)). The ligands and integrins
on the monocytes bind to the adhesion molecules expressed on the endothelial cell surface and
transmigrate into the subendothelial space. The monocytes then transform into macrophages and bind
to highly-oxidized low-density lipoprotein (LDL) to form foam cells. The death of foam cells leaves
behind a growing mass of extracellular lipids and other cell debris along with the accumulation of
smooth muscle cells, which later form fibrous plaques. Vulnerable plaques with thin fibrous caps
have high risk to be ruptured and form thrombus inside the lumen.(Lusis 2000)
|
|
 Fig 2b.
The range of normal shear stress in an artery is between 10-70
dyne/cm2, and the time averaged shear stress for a healthy artery is around 12-15
dyne/cm2 (AM Malek 1999). In contrast, the magnitude of the shear stress in the
atherosclerosis-prone regions is significantly lower (±4 dyne/cm2). Detailed studies
have been performed to investigate the influence of the magnitude of laminar shear stress on
endothelial cells, but only at particular shear values that are considered as high
(12 or 20 dyne/cm2) and low (2 or 4 dyne/cm2) shear stress. The difference
in magnitude usually results in opposite changes in endothelial cell functions, for example low
shear stress (2 dyne/cm2) upregulates VCAM-1 (S Mohan 1999) expression while high stress
(20 dyne/cm2) attenuates it (JJ Chiu 2004). However, the change in EC functions in the
intermediate shear stress range remains unclear.
Fig 2b.
The range of normal shear stress in an artery is between 10-70
dyne/cm2, and the time averaged shear stress for a healthy artery is around 12-15
dyne/cm2 (AM Malek 1999). In contrast, the magnitude of the shear stress in the
atherosclerosis-prone regions is significantly lower (±4 dyne/cm2). Detailed studies
have been performed to investigate the influence of the magnitude of laminar shear stress on
endothelial cells, but only at particular shear values that are considered as high
(12 or 20 dyne/cm2) and low (2 or 4 dyne/cm2) shear stress. The difference
in magnitude usually results in opposite changes in endothelial cell functions, for example low
shear stress (2 dyne/cm2) upregulates VCAM-1 (S Mohan 1999) expression while high stress
(20 dyne/cm2) attenuates it (JJ Chiu 2004). However, the change in EC functions in the
intermediate shear stress range remains unclear.
|
|
 Fig 3.
The flow chamber master (Fig. 3a) consists of two major
components: the linear shear stress flow chamber and the vacuum channel network. The spider web-like
pattern is a network of vacuum channels that helps sealing the flow chamber onto the cover slips
surface, where cells were seeded on. The final flow chamber is transparent to visible light and can
be placed under a microscope to observe cell behavior during the shear flow experiments. The flow
chamber pattern in Fig. 3a was first created using computer software (Freehand, Macromedia)
and printed on a transparency that served as a photo-mask (Fig. 3b) in UV-photolithography
to generate the master mold. In this procedure, a thin layer of photoresist was spin-coated onto a silicon
wafer and exposed to UV light through the photomask. Then a developing reagent was applied to dissolve
the unexposed regions. The resulted bas-relief structure functioned as a master for fabricating
poly(dimethylsiloxame) (PDMS) molds. The surface of silicon/photoresist master was treated with
fluorinated ailanes to prevent irreversible bonding to PDMS. A mixture of PDMS prepolymer and curing
agent was poured onto the silicon master and cured. The PDMS is then peeled off the master and produces
the replica of the flow chamber design.
Fig 3.
The flow chamber master (Fig. 3a) consists of two major
components: the linear shear stress flow chamber and the vacuum channel network. The spider web-like
pattern is a network of vacuum channels that helps sealing the flow chamber onto the cover slips
surface, where cells were seeded on. The final flow chamber is transparent to visible light and can
be placed under a microscope to observe cell behavior during the shear flow experiments. The flow
chamber pattern in Fig. 3a was first created using computer software (Freehand, Macromedia)
and printed on a transparency that served as a photo-mask (Fig. 3b) in UV-photolithography
to generate the master mold. In this procedure, a thin layer of photoresist was spin-coated onto a silicon
wafer and exposed to UV light through the photomask. Then a developing reagent was applied to dissolve
the unexposed regions. The resulted bas-relief structure functioned as a master for fabricating
poly(dimethylsiloxame) (PDMS) molds. The surface of silicon/photoresist master was treated with
fluorinated ailanes to prevent irreversible bonding to PDMS. A mixture of PDMS prepolymer and curing
agent was poured onto the silicon master and cured. The PDMS is then peeled off the master and produces
the replica of the flow chamber design.
|
|
 Fig 4.
The design of the linear shear flow chamber was adapted from
Usami's work (S Usami 1993) based on Hele-Shaw flow theory. By setting the side
walls of the flow chamber to be coincident with the streamlines of a two dimensional
stagnation flow and making the end of the channel shaped to match the iso-potential
lines, this flow allows the wall shear stress to decreases linearly along the center
line of the channel (Fig. 4a). The shear stress at the wall
Fig 4.
The design of the linear shear flow chamber was adapted from
Usami's work (S Usami 1993) based on Hele-Shaw flow theory. By setting the side
walls of the flow chamber to be coincident with the streamlines of a two dimensional
stagnation flow and making the end of the channel shaped to match the iso-potential
lines, this flow allows the wall shear stress to decreases linearly along the center
line of the channel (Fig. 4a). The shear stress at the wall
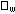 decreases linearly across the flow chamber.
Along the center of the flow chamber the shear stress is a function of viscosity decreases linearly across the flow chamber.
Along the center of the flow chamber the shear stress is a function of viscosity 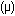 ,
volumetric flow rate (Q), channel height (h = 100 ,
volumetric flow rate (Q), channel height (h = 100 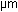 ), entrance width (w1 = 2 mm),
channel length (L) and position (x). ), entrance width (w1 = 2 mm),
channel length (L) and position (x).
Fig. 4b shows the setup of the in vitro flow experiments on human aortic endothelial cells (HAECs). The cell cover slip is temporarily bound to the PDMS flow chamber by vacuum. Flow medium was infused into the chamber by a syringe pump to create a known, spatially varying shear field. |
|
 Fig 5.
After applying a shearing stress for four hours to the HAECs, the PDMS
flow chamber was carefully removed from the cover slip without damage to the cell monolayer. Then
fluorescently-labeled antibodies were applied and a series of fluorescence images were acquired at
different regions of the flow channel that correspond to different shear stress levels (Fig. 5 c-e).
The mean pixel intensity for each region of interest was calculated from 8-10 images. The fluorescence
intensity in the images is positively correlated the level of protein expression on the endothelial
cell surface. The result of VCAM-1 expression represented in fluorescence pixel intensity is plotted
with respect to shear stress in Fig. 5b. With increasing shear magnitude, the level of
VCAM-1 expression gradually decreases (Fig 5c), and the change is more significantly
when shear stress is less than 6 dyne/cm2. Compared to the static control, dramatic increases in VCAM-1
are found when the shear stress is lower than 5 dyne/cm2. Similar results for E-selectin and
ICAM-1 expression are found in Figs 5d,e, respectively.
Fig 5.
After applying a shearing stress for four hours to the HAECs, the PDMS
flow chamber was carefully removed from the cover slip without damage to the cell monolayer. Then
fluorescently-labeled antibodies were applied and a series of fluorescence images were acquired at
different regions of the flow channel that correspond to different shear stress levels (Fig. 5 c-e).
The mean pixel intensity for each region of interest was calculated from 8-10 images. The fluorescence
intensity in the images is positively correlated the level of protein expression on the endothelial
cell surface. The result of VCAM-1 expression represented in fluorescence pixel intensity is plotted
with respect to shear stress in Fig. 5b. With increasing shear magnitude, the level of
VCAM-1 expression gradually decreases (Fig 5c), and the change is more significantly
when shear stress is less than 6 dyne/cm2. Compared to the static control, dramatic increases in VCAM-1
are found when the shear stress is lower than 5 dyne/cm2. Similar results for E-selectin and
ICAM-1 expression are found in Figs 5d,e, respectively.
|
|
 Fig 6.
Leukocyte recruitment is mediated by the expression of adhesion
molecules on the surface of the endothelium. Recruitment can be separated into three steps:
rolling, arresting (or adhesion) and transmigration (Fig.6b). Leukocyte rolling
along the endothelial surface is mediated by selectins which bind to carbohydrate ligands on the
leukocytes. Firm adhesion of monocytes to the endothelium can be mediated by the integrin VLA-4
that interacts with VCAM-1 on the ECs. Monocytes isolated from human blood were defused over the
endothelial cells at 2 dyne/cm2 after four hours of shearing. A series of image
sequences were recorded at different locations on the linear shear flow chamber (Fig. 6a).
The number of monocytes that were rolling, adhering and transmigrating were counted offline.
Fig. 6c shows the number of monocytes interacting with endothelial cells when the
monolayers were preconditioned at different level of shear stress
(low shear: 2 dyne/cm2; med shear: 6 dyne/cm2 and high shear: 12 dyne/cm2)
for four hours. The variation in the number of monocytes that were rolling and adhering to the EC surface
was positively correlated with the alteration in expression level of E-selectin and VCAM-1 with respect to
shear stress.
Fig 6.
Leukocyte recruitment is mediated by the expression of adhesion
molecules on the surface of the endothelium. Recruitment can be separated into three steps:
rolling, arresting (or adhesion) and transmigration (Fig.6b). Leukocyte rolling
along the endothelial surface is mediated by selectins which bind to carbohydrate ligands on the
leukocytes. Firm adhesion of monocytes to the endothelium can be mediated by the integrin VLA-4
that interacts with VCAM-1 on the ECs. Monocytes isolated from human blood were defused over the
endothelial cells at 2 dyne/cm2 after four hours of shearing. A series of image
sequences were recorded at different locations on the linear shear flow chamber (Fig. 6a).
The number of monocytes that were rolling, adhering and transmigrating were counted offline.
Fig. 6c shows the number of monocytes interacting with endothelial cells when the
monolayers were preconditioned at different level of shear stress
(low shear: 2 dyne/cm2; med shear: 6 dyne/cm2 and high shear: 12 dyne/cm2)
for four hours. The variation in the number of monocytes that were rolling and adhering to the EC surface
was positively correlated with the alteration in expression level of E-selectin and VCAM-1 with respect to
shear stress.
|
|
|
References:
[1] AM Malek, S. A., S Izumo (1999). "Hemodyamic shear stress and its role in atherosclerosis." JAMA 282: 2035-2042. |




